COVID-19 cases in Cuba soared to a record 6,422 on Friday, practically twice the quantity registered only a few days in the past, whereas deaths reached 28 because the island struggles to include the virus in high-transmission areas and within the capital Havana.
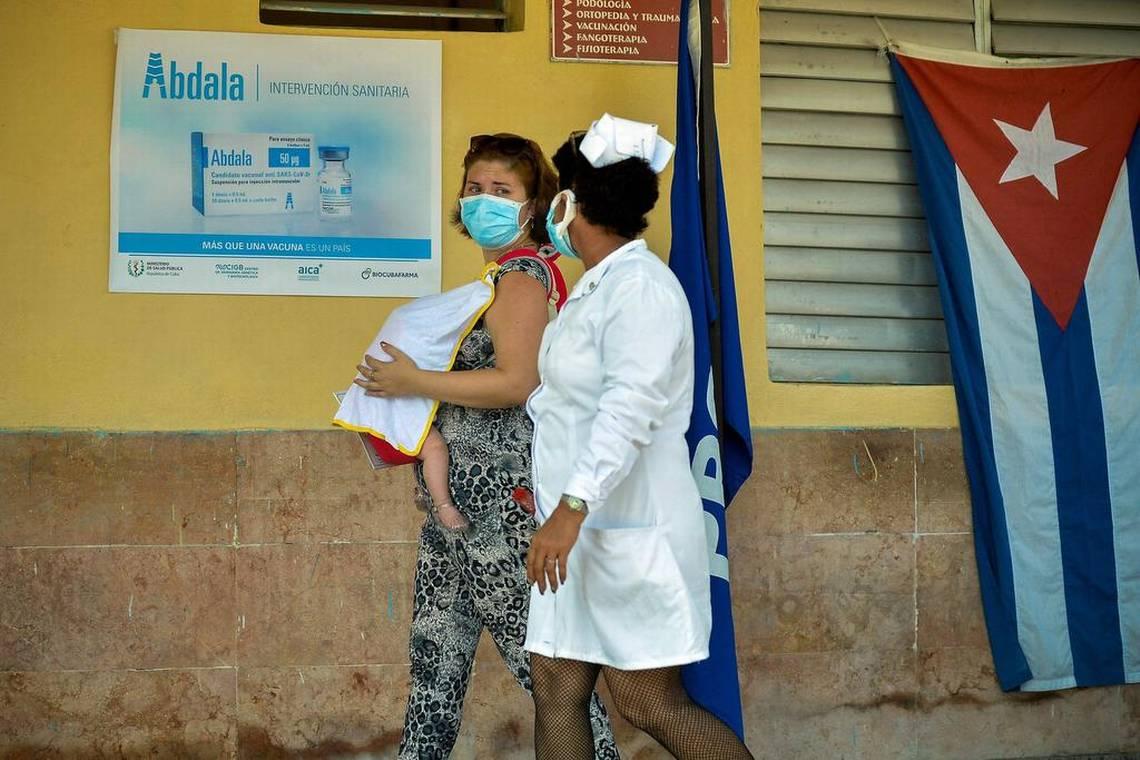
The record comes the identical day that certainly one of Cuba’s 5 vaccine candidates, the three-shot Abdala, obtained native regulatory approval for emergency use, which may assist velocity up the vaccination marketing campaign within the island that determined to make its personal pictures reasonably than purchase vaccines from different international locations.
About half of all cases occurred within the province of Matanzas, the present epicenter of Cuba’s pandemic, the place the seaside resort of Varadero reopened to tourism late final yr, which probably elevated transmission, native well being authorities mentioned.
“I don’t have to say that this is the worst day for Cuba since the start of the pandemic,” Dr. Francisco Durán, nationwide director of epidemiology, mentioned in a video convention on Friday.
COVID-19 case numbers have been rising steadily since late final yr after Cuba reopened its borders to tourism in November. Total cases because the begin of the pandemic reached 219,000 on Friday and 1,431 deaths have been confirmed, in keeping with the Ministry of Public Health. In all of 2020 Cuba mentioned it had simply round 12,200 COVID-19 cases.
Cuba opted to develop and produce a homegrown vaccine in opposition to the lethal virus, a difficult take a look at for a biotech trade that has suffered from a scarcity of funding and difficulties posed by the tightening of the U.S. embargo lately.
As trials lingered on and rollout plans saved getting delayed, the federal government in May introduced a “sanitary intervention” on account of spiking cases and started vaccinating individuals with the Abdala and the Soberana 02 vaccines, the candidates that had been additional alongside in late-stage medical trials, even earlier than they bought official approval.
Cuba’s drug regulatory authority CECMED mentioned in a press release on its web site Friday that Abdala “complied with all parameters and requirements in terms of quality, safety and efficacy.”
State pharmaceutical firm BioCubaFarma mentioned final month that Abdala had an efficacy price of 92.28% in defending individuals in opposition to severe COVID-19 an infection after the conclusion of stage III medical trials. On Thursday, the corporate mentioned that the two-shot Soberana 02 vaccine, when supplemented with a booster referred to as Soberana (*28*), was 91.2% efficient in lowering severe sickness attributable to the virus, in keeping with outcomes of medical trials.